- Ron Rosedale – Fiber – Need It or Not?
- The American Gut Project
- Justin Sonnenburg – Fiber & “Starving our Microbial Self”
- Alberto Martin – Carbohydrates and Colon Cancer
- Eric Westman MD – Low Carb, Health & the Microbiome
Up next, we’ll talk with Justin Sonnenburg of Stanford University about his recent paper in the Journal Cell, titled, “Starving our Microbial Self.” Sonnenburg does ground-breaking research using germ-free mice, where he implants in them various species of gut microbes to see what happens. He observes that when mice eat a typical chow such as cracked corn, it feeds not just calories to the mice, but fibers to the microbes in the colon. As the microbes munch those fibers, they produce beneficial byproducts, such as vitamins and fatty acids, that in turn promote “our” health. That includes the health of our gut cells, which depend on fatty acids to stay healthy. Sonnenburg warns the typical Western diet, which is low in microbiota accessible carbohydrates, results in hungry microbes that don’t make enough fatty acids to feed the gut. And if those “beneficial” microbes get hungry enough, Sonnenburg warns that they can even start “eating” the gut. Justin also points out that he’s a bench scientist who works with mice. He has not studied ketogenic diets in those mice. And, he’d like more conversations with clinicians, including those who use ketogenic diets therapeutically. Here’s Justin Sonnenburg.
(note – this transcription includes paraphrasing)
Justin Sonnenburg – This will be an interesting conversation. I’m looking forward to it.
SHELLEY – Me, too! There’s already a lot of conjecture about what counts as a healthy gut and what does not, and a lot of opinion, already, about what ways to eat support a healthy gut and which ways are bad. Do you think this is appropriate or somewhat premature?
JUSTIN SONNENBURG – That’s a very good question. My wife is a senior scientist in my lab and we run the lab together. We’re writing a book for the general public to demystify the science of the microbiota to some extent, and to make sure when people are reading news articles, they can have a good foundation of knowledge. Also, we want to talk about the practical aspects of what we’re learning. We have two daughters, age 8 and 6, and we realize that how we’re raising them, what we’re learning about the microbiota is influencing what we do. We thought that sharing the perspective is very important, with the idea that there are a lot of caveats and it’s still very preliminary. But if we wait until the point that every single double blind controlled study needed to actually make a claim has been done, it’s going to be decades down the road. I think it’s not necessary to wait until that moment when all the arrows are pointing in the same direction.
SHELLEY – Well, I can’t help it! I’ve got to ask, what is your family eating?
JUSTIN SONNENBURG – Primarily a plant based diet. We’re not completely vegetarian, but many nights we sit down to dinner and realize that what we’re eating is vegan. We try to limit meat. We eat some fish, we eat some red meat, we eat some chicken. But mostly plants. We eat a lot of whole grains, a lot of legumes, a lot of vegetables and a lot of fruit.
SHELLEY – That’s a background of what your family is doing, based on your research. Let’s go ahead and talk about who are you and what you do.
JUSTIN SONNENBURG – My name is Justin Sonnenburg at Stanford University School of Medicine. Specifically, my lab focuses on understanding the gut microbiota. This is the complex community of microbes that dwells within every person in the distal portion of the digestive tract. We have been studying this at Stanford about the past 6 years.
SHELLEY – This is in the colon – the gut.
JUSTIN SONNENBURG – Yes. We have bacteria on every surface of our body. But the vast majority of microbes are found in the colon, the part furthest away from the stomach.
SHELLEY – It’s the very end of the line.
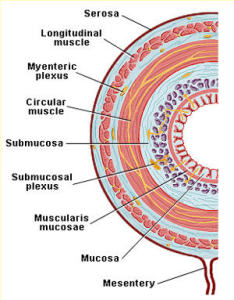
JUSTIN SONNENBURG – Exactly. If you look at the architecture of it, it doesn’t have the feathery villi like the small intestine to maximize nutrient absorption. Instead, our distal gut . . .It takes up a lot of water and it takes up a lot of fermentation products from the microbes like short chain fatty acids.
Add to . . . Germ-free Mice
SHELLEY – Your research has been looking a lot at mice that don’t have germs to start with, and then you add some germs.
JUSTIN SONNENBURG – Yes. The microbiota is a complex microbial community. There are probably around 1,000 bacterial species in our colons, and on top of that, there are a lot of bacterial viruses such as “phage”, and eukaryotes, in other words, parasite like microbes and it’s a really complex system. There are members of the archaea, so this is a complex ecosystem. One way of studying it is by surveying the community in its vast complexity by sequencing the genes of the microbes, or by cataloguing all the species in these complex samples. The other way of studying it is the way we have spent a lot of time studying the microbiota, starting out with a mouse that lives in a sterile environment, and then we add 1 to 4 different microbe species to the digestive tract and see how these affect the other species, and also the host. We can also add a human microbiota to the mouse, and so we have a mouse harboring human microbes and do experiments to look at the complex microbiota.
SHELLEY – In that case, when looking at a human microbiota, you add in a thousand species.
JUSTIN SONNENBURG – Yes, a stool sample to colonize with a thousand species.
SHELLEY – It looks like, at many universities, the focus is on microbes that do not include yeast. Because yeast is a different family and there’s a different way to study it. Do you study yeasts in addition to bacteria?
JUSTIN SONNENBURG – We’re not. You’re right. Most of the emphasis for studying the microbiota in experimental models is on bacteria right now. There have been some studies of yeast as a eurkaryote. Particularly in immune compromised host animals, mice, and looking at how yeast can cause infections. But a lot of the diversity and complexity of the microbial hasn’t worked its way completely into experimental models for understanding. Part of the is because we’re at the very early stages of what we study. Right now investigators are focusing on the components that are the most abundant in the gut, or working on the ones for which there is the most foundation of knowledge, and that is bacteria.
Microbes and the “Mucus Gel”
SHELLEY – You’re interested in the microbes in the mucus gel that’s right next to the human cells that are the lining of the gut, as opposed to the microbes in what’s going down the center of the pipe.
JUSTIN SONNENBURG – The mucous gel is important in at least two respects. One, it creates a barrier between the microbial community and host tissue of the intestinal epithelium, so that we don’t have too much contact between microbes and the intestinal epithelium, and that’s important because we don’t want too much of an immune response which we would get if the bacteria were to close. So the mucus makes a barrier but it also feeds the microbes during times when their other food, which is dietary carbohydrates, such as dietary fiber or Microbial Accessible Carbohydrates (MACs) are not abundant. So mucus plays an exclusionary role by keeping microbes bound to the center of the gut, or the lumen, but also helps feed the microbes at a time when they need sustenance.
SHELLEY – Does the current poo samples collected by most researchers show what’s happening to microbes in the mucous gel?
JUSTIN SONNENBURG – If we look at stool samples, we can get a good idea of the microbes residing in the colon. There have been studies looking at this and the stool sample reflects very nicely the composition of the microbes living in the colon. So we can have a good idea of who these microbes are. If we perform the right assays and measures, we can tell whether those microbes are eating mucous or dietary carbohydrates, and there are methods being developed, some in our lab, to understand more about barrier function in the mucous gel and the epithelium. It’s early days in those studies.
SHELLEY – So being able to tell exactly what’s happening with microbes that live right next to or even in the mucus gel, that’s not as easy to do with a poo sample right now.
JUSTIN SONNENBURG – Exactly.
What “Starves” our microbial self?
SHELLEY – In your recent paper about “Starving the microbial self” you begin by writing, It is possible that the Western microbiota is actually dysbiotic. How would you rank the likely suspects for a sick gut?
- Antibiotics
- Processed carbs that lack fiber
- Sugar
- Fat in general
- Inflammatory Fats
- Inflammatory Foods
- Parasites and other invaders
- Protein
- Lack of vegetables
- Lack of fruit
JUSTIN SONNENBURG – I think a lot of these are in play. The ones that I think are foremost in peoples minds for ways we starve the microbial self are antibiotics, and the lack of fiber, and also, things like birth through C-section, and formula feeling instead of nursing, because human milk has some important compounds that help promote a healthy microbiota. All the other things you’ve listed are candidates. And in terms of ranking them, it’s very preliminary to do.
SHELLEY – So antibiotics, processed carbs, and all that you have mentioned . . . all those things could affect the microbiome.
JUSTIN SONNENBURG – Because these communities are so tied to diet, if you get too extreme in any area, you will likely impact the microbiota in some ways. and there could be negative health consequences for that. The inflammatory foods, if the host is inflamed, it can make more problems in the microbiota. So increased inflammation can be caused by the microbiota and then can come back also hurt the microbiota.
 Fiber – Grains & Beans or Kale and Onions?
Fiber – Grains & Beans or Kale and Onions?
SHELLEY – Do we need to eat grains and beans to get the fibers you think the micro biome needs, or could kale and onions do it?
 JUSTIN SONNENBURG – We totally don’t know what fibers are better than others and if there are certain fibers we should avoid. For instance kale and onions, in terms of where our knowledge is right now, they might be just as good as grains and beans. It may be at the end of the day they turn out to be better. Or worse. We don’t know right now. Certainly fibers from fruit is a good thing. What’s clear overall is that in modern countries, we’re eating way less fiber than we did on the ancient savannah, and this is likely not a good thing.
JUSTIN SONNENBURG – We totally don’t know what fibers are better than others and if there are certain fibers we should avoid. For instance kale and onions, in terms of where our knowledge is right now, they might be just as good as grains and beans. It may be at the end of the day they turn out to be better. Or worse. We don’t know right now. Certainly fibers from fruit is a good thing. What’s clear overall is that in modern countries, we’re eating way less fiber than we did on the ancient savannah, and this is likely not a good thing.
SHELLEY – Is is mainly that we don’t eat enough resistant starches, or it combined with all the other lifestyle messes we get into?
Microbiota Accessible Carbohydrates – MACs . . . for Mice
JUSTIN SONNENBURG – A lot of people use the term resistant starch, and this is certainly a type of MAC (Microbial Available Carbohydrate). But it’s a very specific MAC and the spectrum is much larger than that. Just so people keep in mind that there’s a huge diversity of these carbohydrates in food that have the potential to feed the microbiota. Indeed, most starches are high glycemic load and that’s a problem. But it’s a very complex factor and it’s probably multi-factorial in terms of moder plagues.
SHELLEY – You were saying that the starch in a grain may not be as important to what ends up in the gut as the fibers in a grain.
JUSTIN SONNENBURG – Yes. And the problems with our health probably extend well beyond lack of Microbiota Accessible Carbohydrates – MACs. We’re probably eating way to many refined starches and foods with high glycemic loads. We’re not exercising enough, we have chronic stress. That there’s a lack of dietary fiber or MAC is also something that should be on people’s radar.
SHELLEY – To study MACs and microbes, what kind of diet do you feed your mice? Here’s a list:
- Typical mouse chow (High in MACs fiber?) from grains, beans and roots, such as Ground corn, dehulled soybean meal, dried beet pulp, ground oats)
- Western style diet (low in MACs, high in white powdered sugar, flour and cornstarch and also a fair amount of fat (around 30%, and high in Omega 6s)
- Very low carb, high protein (around 12% carbs, 70% protein)
- “High fat” mouse diet – Basically 20% protein from milk, 40% carbs – mainly sugar and 40% fat, mainly from lard, with a little soybean oil mixed in.
- Very high fat, very low carb, ketogenic mouse chow. Over 70% fat, under 10% carb, not much protein.
JUSTIN SONNENBURG – We have a spectrum. We have probably 15 or 20 different custom diets that we feed to the mice. They range from very high fiber, high MAC typical mouse chow that appears to maintain a lot of diversity in the gut microbiota and results in a lot of production of short chain fatty acids produced by the microbes. We also use several types of high fat diet. This includes the Western style, which is also high in simple starches and simple sugars. We have high protein diets. We have diets that are high in inulin as the only dietary fiber. So we’ve studied many many different types of diets and their impact on the microbiota.
SHELLEY – Is there any diet that you have not studied?
JUSTIN SONNENBURG – We haven’t done a 70% protein. I think we’ve done up to 40% protein. We have . . . high fat mouse diet (50% fat, 40% sugar) – we have something that’s like that.
SHELLEY – Have you done something that’s so low in carbs, it’s 12% or less carbs
JUSTIN SONNENBURG – We have not.
SHELLEY – Have you done Omega 6s versus Omega 3s in the fat.
JUSTIN SONNENBURG – That extends beyond our scope.
SHELLEY – How about a very high fat, ketogenic mouse chow? Meaning as high as 70% or 80% fat . . . perhaps even more?
JUSTIN SONNENBURG – We’ve studied that in several different contexts. More than the studies we’ve worked on, there’s a very beautiful study published in Nature, Devkota et al that compared the impact of saturated fat versus polyunsaturated fat on the microbiota and showed very nicely there was a bile salt dependent impact on the microbiota that actually made the mice consuming the saturated fat diet more prone to colitis in genetically predisposed hosts, so some connection potentially there between saturated fat and inflammation in the colon.
SHELLEY – That’s been in mouse studies and . . . I didn’t quite hear. Have you done a ketogenic mouse chow that’s over 70% fat under 10% carb roughly, and not much protein at all.
JUSTIN SONNENBURG – We haven’t done this. We’ve been focused so much on understanding the dynamics dietary fiber utilization that we haven’t extended into that realm.
High Fiber and . . . Colon Cancer?
SHELLEY – Did you get a chance to look at Alberto Martin’s new study in Cell?
JUSTIN SONNENBURG – Yes I know that work. That’s an interesting paper. It’s first important to acknowledge that many of these studies that are being cited and written about are very preliminary. Most are very good studies, including this one. It will be years to determine if the results are reproducible in mice by other groups – that’s always important, and number 2, applies to humans. We must keep in mind not to over interpret very early reports.
SHELLEY – Can you give a quick summary about the gist is of Alberto Martin’s paper?
JUSTIN SONNENBURG – They nicely show butyrate in the colon, one of the products of fiber utilization — it’s a short chain fatty acid, that I should say, has been linked, and the authors have acknowledged this, in many other studies, to attenuating inflammation.
SHELLEY – Meaning reducing inflammation.
JUSTIN SONNENBURG – Reducing. Yeah. In this model it appears to promote cell proliferation. And indeed, other groups have shown that as well.
SHELLEY – This is in mice bred to be prone to colon cancer, one of the more common kinds of colon cancer in people.
JUSTIN SONNENBURG – Exactly. So these were genetically predisposed hosts. I think the big picture here is that biology is complex. The microbiota is complex. Our genomes are complex and individual. And what may be true for one person and what may not be true for most people, 90% or 99% of the people, may not be true for everyone. It’s possible, for instance, people who are susceptible to colorectal cancer, or perhaps other diseases, need to be cautious. I think this speaks to the importance of personalized medicine. Understanding your genome and what you need to be aware of. There’s abundant data indicating that short chain fatty acids like butyrate have many beneficial effects, influencing both the immune system and metabolism. And many of our modern plagues and diseases have at their basis inflammation and disregulation of metabolism. The story is complex and it’ll take a while to weed through this.
 SHELLEY – Eating butter . . . . If someone’s eating a low carb, adequate protein, high fat diet that promotes ketosis, in that case, the liver makes, basically, its own butyrate. Since butyrate is a fuel that feeds human gut cells . . . Could the betahydroxybutyrate made by the liver help out the intestine and keep it healthy, and help out the gut microbes there?
SHELLEY – Eating butter . . . . If someone’s eating a low carb, adequate protein, high fat diet that promotes ketosis, in that case, the liver makes, basically, its own butyrate. Since butyrate is a fuel that feeds human gut cells . . . Could the betahydroxybutyrate made by the liver help out the intestine and keep it healthy, and help out the gut microbes there?
JUSTIN SONNENBURG – It’s an interesting idea. I don’t know if there’s any data that speaks to that and I’m not sure about evidence that hydroxybutyrate can bind to the free fatty acid receptors, or to GPR 41 and 43. that many people are showing are so important to fatty acid utilization. So interesting idea but I’m not sure about that.
SHELLEY – How about just eating something that has butyrate in it. Doesn’t butyrate have its name from butter.
JUSTIN SONNENBURG – One of the things that’s really not clear is whether the site of butyrate absorption is important. There are some studies that have shown that you can feed a short chain fatty acid by mouth, and that can mimic production of in the colon. Other studies have shown you have to administer the short chain fatty acid rectally or deliver it to the colon through some chemical means.
SHELLEY – That’s what Alberto Marin had to do, because he couldn’t overload the colon with butyrate by simply feeding that fatty acid to the mice. Instead, either he had to feed the gut microbes getting enough dietary fiber to make the butyrate themselves, or he had to use suppositories to insert them into the colon. You’ve mentioned some concerns about whether a lower fiber diet might cause an increase of inflammatory conditions. But in reports about high fat ketogenic diets, clinical researchers working with humans have pointed out that low-carb diets can be therapeutic for chronic digestive disorders, such as Crohns disease and irritable bowel disease. There are reports that these tend to go into remission on a high fat, low carb diet. Have you heard that, too?
JUSTIN SONNENBURG – I haven’t. So this is . . . and I’m trying to be better about this. But one of the real big problems of being a basic scientist is you have almost no contact with the clinic and very little contact with the clinicians. We’re separated from that environment, but I’ll have to look into that.
SHELLEY – Eric Westman of Duke University has pretty consistently said those are some of the first symptoms that people report changing when he puts them a low carb, high fat diet.
JUSTIN SONNENBURG – Interesting.
SHELLEY – How about Loren Cordain’s concerns, that many of the foods recommended as resistant starches or high in fiber, such as beans and lentils and whole grain products, contain lectins and other irritants that don’t always cook out of food, and can be irritating and may cause leaky gut all on their own.
JUSTIN SONNENBURG – First of all, I really like the spirit of the argument because thinking to our evolutionary past is really important part of trying to make sense of the situation we’re in now. Couple of things we have to keep in mind. One is, how rapidly are humans evolving and how long ago were certain foods introduced, and how likely is it that we’ve adapted to certain things. For instance, how likely have we adapted to the ability to consume lactose by early tribes of humans that started drinking milk. Concerning the relation of lectins to leaky gut, I’m not aware of any data. I’m aware of lectins. I studied them in graduate school. It’s clear that lectins can be toxic in certain situations. Whether they play any role in digestive disease – if there’s any data to that effect, I’d love to see them.
SHELLEY – What else would you like to talk about?
Butyrate from the Bloodstream?
JUSTIN SONNENBURG – I would love to hear your theory on beta-hydroxy butyrate.
SHELLEY – Well, it’s not my theory – it’s what some clinicians say who work with high-fat diets. And . . . I’m curious about the high fat diets that are not high in protein. They’re low carb – lower than 80 grams of carbs a day, adequate protein diets. Ron Rosedale, who’s a clinician, said his guess is that gut cells are like any other kind of cell. You want to feed them, but you don’t want to feed them too much. If you feed them too much, it switches them from maintenance and repair to proliferation mode, which can promote cancer. Having such high fiber in the diet that the gut microbes produce loads of butyrate, he suggests, can become a cancer promoter in the colon because gut cells are one of the few tissues in the body that really loves to gobble up fatty acids. If they have a superabundant amount of fatty acids, it could keep them switched over more toward dividing, which is more cancer territory, than keeping them in maintenance and repair. Especially in an environment where the insulin levels are not kept low and other inflammatory markers are somewhat high.
Modern Living – Would 100 grams of fiber daily be okay?
JUSTIN SONNENBURG – This is something that’s really bothered me, and we’ve talked about it a lot in my lab. Hunter gatherers in some regions of Africa are eating somewhere upwards of 100 grams of dietary fiber a day. There are studies of traditional agrarian groups that produce way more short chain fatty acids in their guts than we do and are eating more fiber. I think some people argue that their life expectancy is short enough that things like colorectal cancer are difficult to gauge. I would like to get more data about whether or not these traditional communities are protected and don’t exhibit the colorectal cancer rates that we exhibit or it’s just not possible to assertion due to lifespan.
SHELLEY – Whatever’s happening with their fiber intake, because they are hunter gatherers, I’ll bet their insulin levels are lower and their blood sugars are lower than the typical American’s.
JUSTIN SONNENBURG – Exactly. So this is the other part that you hit on that I think is important. We trying to recapture a diet in a time that’s very different. We live in a nutrient rich environment, and we’re not struggling to find calories and going through periods where we don’t have a lot of calories. It may be that. As modern people, we may have to be cautious about moving back to a very high fiber diet. And that’s something that we capture at the end of “starving our microbial selves.” There’s a line in there that says are there going to be deleterious consequences of trying to recapture aspects of an ancient diet in such a different environment context as the modern world. And that’s something I think we need to be very aware of.









1 comment for “Justin Sonnenburg – Fiber & “Starving our Microbial Self””