(NOTE – This transcript includes paraphrasing)
LISTEN (38 Minutes)
SHELLEY – Ron Rosedale – These days, many advocates of a higher fiber, more complex carbohydrate diet contend that our microbiome needs starches that are slow to digest, so that those undigested foods can make it deep in the gut, where they can nourish beneficial microbes. They caution that a low-carb, high fat, adequate protein diet does not provide enough nourishment for our bodies to maintain essential and beneficial gut microbes. Do you agree?
RON ROSEDALE – The simple answer, if I had to give one, would be no. We certainly don’t need resistant starches. What would be a better food in that vein would be resistant fibers. Resistant starches do resist digestion in the upper digestion, and then in the gut, bacteria will partially turn those resistant starches into fatty acids. But being a resistant starch doesn’t mean that none of that starch will be digested into glucose. The portion of a resistant starch digested into glucose will be detrimental, and there’s nothing good about that, whether it’s called safe starch or resistant starch. All that would be the story for another day. You can find many arguments and debates about the so called safe starches and the detriments they may cause, regarding increases insulin and blood glucose. Rather than “safe” starches (such as beans, for instance), a person would be better off to eat undigestible starches (such as insoluble fiber extracted from fruit) . . . at least undigestible to what is considered “us” and more digestible to microbes, and focusing on the fibers instead of the starches would be less likely to raise insulin and leptin.
 Microbe-Eating Animals
Microbe-Eating Animals
SHELLEY– On this concept of insoluble fiber versus slow-to-digest, or “resistant” starch – you’ve pointed out in previous discussions that termites are an example of an animal that doesn’t really “eat” starches. Instead, they eat resistant fibers that the microbes in their guts digest. And then the termites eat the byproducts of that microbial digestion, plus they eat the microbes.
RON ROSEDALE – Cellulose is what the bacteria in stomachs of termites eat. Termites don’t “eat” the wood. They farm the bacteria that eat the wood. Cows do the same. They don’t “eat” grass. They farm bacteria that can eat the grass, and then the cows eat the protein of the microbes and the fatty acids. So they “eat” a protein and fatty acid diet. Cows feed the grass to the microbes. They farm the microbes just like we farm the cows.
SHELLEY – Well, cows in feedlots get grain that, if it were cooked, we humans could also eat. But even when cows are eating grain, unlike us, the starchy part doesn’t go directly into being metabolized into sugars as much as those calories does in us. Instead, the cows have evolved to use their enormous and several stomachs to ferment that grain from the get go to feed the microbes. But that’s still stretching it a bit – grains are an unnatural diet for a cow. In pasture fed cows, they’re eating grass that we humans would definitely find undigestible.
RON ROSEDALE – The microbes are a key to a cow’s digestion. The entire image of what eating “is” has to be revised, and the entire concept of health needs to be revised so that people understand that bacteria are part of us. Regarding “eating” . . . where do we get oxygen from? We get it from plants. But that makes the line fuzzy between, “What’s an animal and what’s a plant?” Being a vegetarian is not really being a vegetarian. The vegetarians among us are eating the microbes inside of “us” that eat the plants. In addition to changing our concept of “eating,” we need to revise what it means to be healthy. For instance, many proponents of high fiber diets argue that if you eat foods that increase butyric acid in the gut, you’ll be more healthy. And that’s one of the major reasons for eating resistant starches, to feed microbes something that makes the microbes produce more butyrate within the gut.
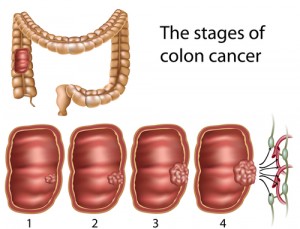
Moderately High Butyrate Levels MIGHT Fuel Gut Cancer
But it turns out, when you increase butyrate in the gut, you greatly increase the risk of gut cancer. That’s being shown in new study or mice and colon cancer, by Alberto Martin in the journal Cell. His study is a reminder that what’s so-called “good” for the gut — meaning butyric acid for fuel — well, if you’ve got any gut cancer hidden inside of you, gut cancer is regular gut cells that need lots of fuel so they can replicate and reproduce. So they need more fuel. By increasing butyrate in the gut you’re giving them more fuel to replicate and that can fuel more potential cancer This kind of paradox is part of the problem involving just what concepts people use to define good health, along with just what “we” are and what “we” are not. After all, cancer cells, they’re human cells. They’re us. But when we think “we’re” being healthy, many times, we’re being healthier for the cancer cells. They require the same nutrients that we do. They are us. But they require more. It takes more nutrients to make babies. Any mother knows that. Can cells divide rapidly. They make lots of babies. When we feed cancer cells extra nutrients, we’re just facilitating their growth. So that has to be thought about. To have better health for “us,” we need to look for differences, between cancer and human cells, and one of the major differences is how to fuel the growth pathways to promote regular cells instead of cancer cells. The MTOR pathway is a huge pathway, important in all life, that integrates the evaluation of nutrient availability and signals for cellular growth and replication. MTOR is stimulated to promote replication through signals that include higher levels of glucose, amino acids and insulin, and all these things tend to go up in a high carbohydrate diet, or in a low carb, high protein diet. Both those diets will increase TOR – Target of Rapamycin and increase cellular proliferation and therefor increase cancer.
SHELLEY – You have mentioned that high protein will push MTOR more toward generating cancer.
RON ROSEDALE – Yes, I think I first talked about that in around 2006, and I’m glad to see that concept is getting quite a bit of traction, and people are starting to really look at that pathway now and realize its importance in terms of protein consumption, and many people now are really questioning a high protein, low carb diet. A low carb diet is not just a single diet. There are huge differences between low carbohydrate diets. There’s the difference between a low carbohydrate and a very low carbohydrate diet. A low carbohydrate, high protein diet, and a low carbohydrate, high fat diet. These are all extremely different ways to eat, and each one has very different consequences when it comes to cancer and diabetes and things like that.
What about Butter?
SHELLEY – Let’s talk further about those diets in a moment. But . . . getting back to the microbiome and how gut microbes produce more butyric acid when they’re fed more “resistant” starch . . .. I’m a little troubled that butyric acid might promote cancer, for two reasons. One – I thought cancer was primarily promoted by excess sugars, not by fatty acids. And Two – I thought that butyric acid is something that butter breaks down into, and I like butter.
RON ROSEDALE – Me, too. I love butter.
SHELLEY – When I eat butter, is the butyric acid in butter what promotes these cancers, or are the cancers promoted more by feeding resistant starches to the gut microbes, which then makes the byproduct, butyric acid, right there in the gut?
RON ROSEDALE – That brings up a wonderful point, which is, the human body is a wonderfully complex network. It’s very difficult to see all the interactions. There are a number of factors that will feed into getting cancer. So in other words, if one eats butter . . . and I eat lots of butter . . .
SHELLEY – But, but . . . given Alberto Martin’s new paper about butyric acid and its relationship to colon cancer, aren’t you scared that eating butter will increase your risk of getting colon cancer?
RON ROSEDALE – No!
SHELLEY – Why not?
RON ROSEDALE – For one thing, most of that butter and most of the butyric acid that’s in that butter will not make it into the colon. It’s going to get digested into your body and eaten for fuel directly by your cells long before it gets that far down in the digestion, and especially, if you’re adapted to burning fat for fuel, by following a very high fat, low carb, adequate protein diet. Being adapted to burning fats changes one’s metabolism such that our cells use fatty acids as their primary fuel. In someone adapted to that metabolism, eating butyric acid is going to get better absorbed, early on in digestion. Take me, for instance. I’ve been eating this kind of diet for 25 years. In me or other people who eat this way, butyric acid will get burned for fuel and probably never make it down into my gut. Furthermore, for instance, in the Cell study, the researchers were using mice to study a very common form of colon cancer that’s also found in humans. This form of colon cancer involves mutations and damage to DNA repair mechanisms. So acquiring this cancer in the mice, or in humans, is a multifactorial process that involves mutations in DNA repair, and increase in DNA damage. You’ll find that kind of DNA damage more often when you have increased inflammation, which I don’t have.
The Mice were Sitting Ducks for Cancer
SHELLEY – So in Alberto Martin’s experiment, his team used mice that were specially bred to have a common DNA mutation/damage that increases the risk of cancer – meaning from the get-go, those mice were sitting ducks for cancer.
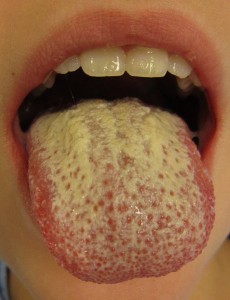
RON ROSEDALE – These mice were kind of sitting ducks for cancer. The extra butyrate then fueled what was already set up in those mice to be cancerous. In people, what can get set up to increase DNA mutations is a going to be higher carbohydrate diet. That means, by the way, that if you eat resistant starches (such as high-fiber grains, yams, potatoes, beans and fruit), you’re going to raise glucose in many parts of the digestive tract, plus you’ll raise it in the bloodstream that feeds the gut. So, you’re going to get the promotion of candida, for instance – yeast – in the gut. That’s one of the most common forms of dysbiosis in the gut.
What about the Yeast?
SHELLEY – Yeast . . . excess yeast! We’ll get back to the mice that got cancer from having “too much” butyric acid in their gut. But first, about this yeast — candida has been known for a long time to break down the intestinal barrier and make for a leaky gut.
RON ROSEDALE – Candida makes for leaky gut, increasing inflammation, stressing the immune system of the gut. It was really the idea of candida in the gut that prompted Steve Barrie to form Great Smokies Lab 25 years or so ago, which was one of the first major labs to study nutritional medicine, and they specialized in microscopic examination of stool and the relationship between gut health and regular human health. Great Smokies was started by the idea that candida caused inflammation of the gut and was detrimental to human health. In fact, one of the first applications of a very low carbohydrate diet 20 some years ago was to cut off fuel for the continual growth of candida in the gut. To try and improve gut health and reduce gut disease, and candida overgrowth in particular, a very low carbohydrate diet was recommended at the time.
SHELLEY – In the new research studies of the microbiome, often yeasts are not part of what researchers even look for. In research labs, most of the special assays that can find the thousands of different microbial signatures in the gut are calibrated to look at the DNA from bacteria and archaea, but not eukaryotes. Eukaryotes are the family of life that includes, among other things, us, pine trees, and yeasts.
RON ROSEDALE – Exactly. Not keeping yeast in mind in these new experiments is a major problem. The bacterial component of the gut micro biome is critical, but you certainly can’t discard what’s been known for a quarter century or so, or longer, regarding yeast.
SHELLEY – So you’re saying, what the heck about the yeast?
RON ROSEDALE – Yeah! What about the yeast? Which has been long known to play a huge role in inflammation and gut health, and also has been known to play a huge role in just what other organisms coexist in the gut biome. When people eat carbohydrate, they are fueling the growth of candida in their gut.
SHELLEY – One argument I hear you saying is that many resistant starches have enough carbohydrate in them . . . that turns into glucose, that if there’s yeast overgrowth in the gut, the resistant carbs might keep feeding the yeasts. And I hear you saying that yeasts in the gut might be nourished by the starches also.
What did the Colon Cancer Mice Eat?
SHELLEY – Now getting back to the Cell study you mentioned, regarding mice and colon cancer. What did they feed the mice?
RON ROSEDALE – What they did in this study in particular, they fed one group a regular mouse chow diet, which is high in fiber and promoted colon cancer in the mice. And they had another group of mice on low carbohydrate diet, which slowed down the rate of colon cancer. I haven’t downloaded the supplement to find out exactly what the mice were eating. But the key to this study was when they took the mice on the low carb diet and added butyrate.
Pure Butyrate Tastes Like Vomit
SHELLEY – How do you add butyrate? I’ve always wondered, so I’ve asked scientists if they could give me some butyrate to taste, and they look at me and say that’s impossible. It’s too volatile, and you wouldn’t like how it tastes anyway. Evidently, it tastes horrible. Worse than vomit. So I can’t picture how you would feed these mice butyrate.
RON ROSEDALE – I don’t know either.
SHELLEY – Have you ever tasted butyrate?
RON ROSEDALE – Not pure butyrate by itself. But however they did it, when the researchers did increase the butyrate being fed to the mice, it’s what proliferated the cancer. On the flip side, there were two approached that helped reduce cancer in these colon cancer prone mice. One was feeding the mice a low carbohydrate diet, and the other was when they fed the mice regular chow – but also gave them antibiotics.
SHELLEY – Okay, help me get this straight. They were feeding one group of mice normal mice chow, which basically tends to be high fiber, resistant fiber stuff such as uncooked, cracked corn and soy . . .
RON ROSEDALE – Normal mice chow, and they developed cancer. In the group of mice that they fed a low carbohydrate diet, OR in the mice who got normal chow, plus were also fed antibiotics, the colon cancer risk was greatly reduced. And there was yet another arm to this study. When they fed the low carbohydrate mice dietary supplements of butyrate, the risk of cancer went back up again. So the researchers concluded it was the metabolism of the carbohydrate into butyrate, within the gut, that was fueling the increased risk of cancer.
SHELLEY – So one thing that slowed down the colon cancer in these mice was feeding them a low carb diet, and one thing that speeded it up was adding in butyrate.
RON ROSEDALE – First it was just a low carb diet, and that reduced the risk of colon cancer. And then when they added butyrate, the risk went back up.
SHELLEY – I’m curious to know just low in carbs they went.
RON ROSEDALE – Right, because even though their low carb diet slowed down the cancer, these mice still got cancer. There’s nothing to say that if their food had gone even lower in carbs, then the cancer incidence would have been even lower. My guess is that it would have been. Getting back to the actual study, it showed that the reduction in cancer, initiated by a low carbohydrate diet was negated when researchers supplemented the mice with butyrate. So the researchers felt it was the butyrate content that was the variable in whether the colon cancer risk was high or low.
Still think eating butter’s okay?
SHELLEY – High butyrate fueled the colon cancer in the gut? I still want to be able to eat butter at the end of this story.
RON ROSEDALE – Oh, yeah. Me, too. This study came out this summer, and I have not reduced my butter one bit.
SHELLEY – Why not? Because all this talk about the dangers of too much butyrate makes it kind of scary. After all, butter is a big source of butyric acid. I know what you said about how a fat-burner is likely to digest the butter so well, little of the butyric acid ends up in the colon. But I almost hearing you wondering, if a person was eating a fairly normal American diet, which is high in carbs, plus butter, would they “burn” the butyric acids fast enough? Or would more of butter’s fatty acids make it down inside the gut and do some dicey things?
RON ROSEDALE – There may be an issue there. After all, we know that the way these mice were set up with mutations to DNA repair are similar to the kind of DNA damage that can grow more likely in people who are on a higher carbohydrate or protein diet. In contrast, the setup that the mice had initially, starting out with mutations in DNA and DNA repair, is going to be minimized for a person who follows a very low carbohydrate, high fat diet to start with. A very low carb, high fat, adequate protein diet is going to reduce TOR – target of rapamycin, signals which promote more cell division, and the low carb high fat diet is also going to keep insulin and leptin low, which will add to the increase of maintenance and repair. We’ve talked in the past about a genetic program that virtually all life has, that can up and down regulate maintenance and repair, and kind of allocate energy towards cellular repair. In life, when there’s an indication that enough fuel and materials are available, there’s a genetic program that says to life, make hay while the getting’s good. That leads to faster cell growth and cell division, which can set the stage for more cancer. In contrast, when “Life” has deemed that there aren’t enough nutrients to make babies or make cells, then the cell will initiate a genetic expression of repair and maintenance that greatly enhances the ability of that “life” to outlive disease and famine. We know that the direction of metabolism toward either cellular replication or toward maintenance and repair is performed by these nutrient sensing pathways such as insulin or more importantly the TOR pathway, which takes insulin into account, such that when you keep TOR low, you increase maintenance and repair, and that includes DNA repair. So on the low carb diet, you don’t have as much damage to DNA from the high glucose, and you increase DNA repair from the nutrient sensing pathways such as insulin and TOR, so you’re not going to be set up for as much cancer in the first place. In a body with better maintenance and repair already, there’s less DNA damage. So any butyrate that makes it down into the gut would be less prone to then fuel cancer. A lot of things have to go “right,” for cancer to really flourish. And conversely, a lot of things have to go right to be healthy.
I think we know a lot about what’s needed in order to be healthy. If people wouldn’t keep messing with it. And by one example of “messing,” I mean the big emphasis today on adding into the diet more, so-called “safe starches” (such as cold potatoes) and even resistant starches (such as beans) to some extent. And I think the problem is also the distinction between what’s good and what’s best. Most people in the United States each a diet that’s so bad, if you make any changes, it’s better. So if you eat resistant starches as opposed to regular starches, will it be better? Absolutely, because you’re not going to produce as much sugar. You might produce half as much digestible carbohydrate from a resistant starch as you would from a regular starch. Might it be that much better to not eat starch at all? Probably. Personally, I’d probably rather not have half the glucose. I’d rather have no glucose. So better does not mean good. Better just means better than bad. And that’s something I think really has to be drilled into people. Because you see so many articles about certain diets that offer improvement to standard health. For instance, diets that lower hemoglobin A1c and cause weight loss. Does that mean that particular diet made a person healthy? Well, just because it improved, doesn’t mean the person is fully healthy. And just because there’s weight loss doesn’t mean the person’s healthier. You lose weight when you get cancer. So these mice in Alberto Martin’s lab that increased their cancer in the butyrate study, the also lost weight. You also lose weight if you eat cyanide. So losing weight doesn’t mean getting healthy, number one. To find health, you certainly need to delve deeper into the science of aging. Certainly mortality can be used as a marker of health. You’re not going to be healthy if you’re dead. If you keep life alive longer, and free of disease, then I think you have evidence of making something healthier.
Why do gut cancer cells thrive on butyric acid?
SHELLEY – Well the life of these colon-cancer prone mice was not longer. They were dead from the cancer sooner, whenever they got mainlined with more butyric acid. But this brings up another question for me. My understanding has been that in studies of cancer, when the microbes are producing butyric acid, that’s actually protective of the lining of the gut, and good for it, in reducing inflammation and cancer. That was my impression. Do you agree with that?
RON ROSEDALE – I agree, to some extent.
SHELLEY – Except that, perhaps those darned, starchy and sugary calories that may increase insulin and leptin and may also increase cancer to some extent.
RON ROSEDALE – And may increase butyrate too much. Because if it increases butyrate to more than what a healthy cell can eat, you might have extra butyrate, and that extra butyrate might fuel the growth of cancer.
SHELLEY – Too much of a good thing.
RON ROSEDALE – Absolutely. It has to be recognized that cancer cells are nice, healthy human cells, and everything that’s good for a regular cell is going to be at least as healthy for a cancer cell.
SHELLEY – That surprises me! I thought that cancer cells tend to have a metabolism that feeds most robustly on sugar. I thought that cancer cells are burning fuel so fast, they can’t afford to use oxygen, and so they use some processes without oxygen that make for a dirtier fuel that produces more toxins, and the best way to do all that dirty, fast-moving stuff is to burn sugar. I didn’t know that butyric acid is also something that feeds a cancer cell.
RON ROSEDALE – It depends on where the cancer is and how fast it’s growing and the type of cell. So in solid cancer tumors, the usual blood supply can’t feed the interior, in other words, those cancerous tumors have outpaced the growth of their blood supply. For tumors like these, the cancer cells must operate more anaerobically, meaning without the oxygen they would have gotten from the blood. So, they will prefer glucose. That’s the case for most types of cancer – the cancer cells will grow faster, if they have more glucose, because glucose is an anaerobic fuel. This is true. And many cancers, even if they have more oxygen, will prefer to burn glucose, because they have a so-called aerobic glycolysis, which is the Warburg Effect.
SHELLEY – Just because growing cancer grows so darned fast and hot.
RON ROSEDALE – Exactly.
SHELLEY – How about this butyric acid.
RON ROSEDALE – Butyric acid fuels a different kind of cancer.
SHELLEY – Hmm, perhaps because intestinal cells, they really like butyric acid? They’re kind of evolved to really think that’s yummy stuff.
RON ROSEDALE – Exactly. And colon cancer, most of the time is not a fast growing cancer. Most of the time, it hasn’t outpaced its blood supply. The cancer itself is more exposed to the air that’s near the gut lining. There’s air right there, so there’s oxygen that can be used to burn fat. So colon cancer doesn’t have the typical problem of lack of oxygen, so it doesn’t have to go into anaerobic metabolism. And it’s not one of these cancers that is so fast-growing that it outpaces the blood supply. So what ought to be the typical image of cancer cells, requiring glucose as their primary fuel, is not 100% true. It’s true for most cancers, but not all of them. It depends on where the cancer is. In this particular case, in this particular kind of cancer, and this particular form of colon cancer, the normal paradigm doesn’t really hold. In this particular case, butyric acid is the preferred fuel of both normal colon cells, and of colon cancer cells.
SHELLEY – These colon cancer prone mice in that study, when they were on a low carbohydrate diet,. Did they eat a high protein diet? Or a high fat diet?
RON ROSEDALE – I don’t know. Typically for research studies using a reduced carbohydrate diet, the calories generally provided by carbs are split between fat and protein. But again, the diet was not really the point of the study. The point of the study wasn’t to show that a low carbohydrate diet was particularly great. The point of the study was to show that when researchers added butyric acid back into a low carb diet, the cancer was again promoted. In other words, lowering the carbohydrate lowered butyric acid production by gut microbes, and that reduced the cancer, but when researchers added butyric acid back in, it negated the benefit of feeding the mice the low carb diet.
SHELLEY – So these researchers decided that that the key take-home for their experiment is that when they fed mice their typical high fiber carbohydrate, “resistant” starch diet, it fed gut microbes that made the butyric acid, and that was creating a lot of butyric acid that would increase the cancer.
RON ROSEDALE – That was the point of doing the experiment in the first place
Leptin and Insulin and Sugar, Too.
SHELLEY – But I hear you saying that lowering the carbohydrates didn’t just produce less butyric acid. It also reduced insulin levels and leptin levels and glucose levels,
RON ROSEDALE – Right.
SHELLEY – Are you thinking the conclusion of these scientists is what you would conclude.
RON ROSEDALE – Well, again, they took mice that had two common mutations that have to do with DNA mutation and a mutation in the ability to repair DNA.
SHELLEY – So it’s going to be a lucky and amazing mouse here that would not eventually get colon cancer.
RON ROSEDALE – In this particular strain of mice, would all get colon cancer, probably. It’s a matter of when and how rapidly. But the reason they set these mice up for cancer is that the two DNA mutations that put them at more risk are commonly found in humans. What I’m saying is that in humans, these DNA mutations are probably going to be more commonly found in people who are eating a high carbohydrate and or a high protein diet. And so those humans are going to be set up for more cancer. And then they’ll fuel the cancer if they have more butyrate, if they have that set up.
SHELLEY – And where will they get the butyrate from?
RON ROSEDALE – Butyrate is going to be found in the diet, but the butyrate that gets down into the colon is going to be from the slow to digest and the insoluble carbohydrates.
SHELLEY – Meaning it’s going to be from the microbes munching away at the high fiber foods and resistant starches that get into the colon.
RON ROSEDALE – Correct, and even if a person were to eat a whole bunch of fiber. Down in the gut, butyrate acid is probably produced less rapidly from insoluble fiber, but I think the speed in which it’s produced is quite critical too, because if it’s produced more rapidly, then it’s going to more rapidly fuel cellular growth than if it’s produced more slowly. So the production of butyrate is going to be produced more slowly from insoluble fiber than from slow-to-digest starches, but it will still be produced.
SHELLEY – But you’re also saying, if someone’s adapted to eating high fat foods, it’s not like they’re flooding their gut with a whole bunch of butyrate. They’re burning most of it as fuel. There’ s not as much getting down into the colon for the microbes to be using.
RON ROSEDALE – That’s part of it, but even more importantly, a person eating a high fat, low carb diet is not going to have these genetic mutations in DNA developing as rapidly. They’re not going to be exposing themselves to high rates of glucose, and they will be upregulating the repair phenotype or genotype.
SHELLEY – And so you think, people who have adapted to not eating a lot of carbs and sugars won’t have high insulin and leptin levels, and their body will stay more tuned to staying in good repair. In that case, it doesn’t matter, having more butter and butyric acid. The cells will still be going more smoothly through their lifespans without rapid multiplication.
Damage vs. Repair
RON ROSEDALE – Life in general, from a very simplistic standpoint, could be seen as a constant battle between damage and repair of damage. If we could repair damage as rapidly as it occurred, we would live forever Most people are looking at health from the damage side of the equation. If you look at healthcare and standard medical care, they’re always looking at damage. If you talk about oxidation and antioxidants. Everyone takes antioxidants because they say oxidation is one of the major causes of damage. And indeed, it does cause some damage, But a lot of times, that damage is actually good for you, For instance, damage in the mitochondria is often a signal for the mitochondria to up regulate repair mechanisms. And many animal studies have shown that if you increase oxidation, at least to some extent, in the mitochondria, these animals will often live longer, because they’ve upregulated repair mechanisms, that have been initiated by the damage to the mitochondria.
SHELLEY – So here we are in that delicate balance area where you want enough, but not too much.
RON ROSEDALE – Exactly. You need to concentrate as much or more, and I would say more, on up regulating repair mechanisms, because we know that’s really plastic. It’s very variable. We know there are really powerful genetic mechanisms that are signaled by nutrients to up regulate our ability to repair damage. That especially includes DNA repair. Our bodies can make and up regulate heat shock proteins, antioxidants. and when this is initiated from inside the cells, it’s more effective, because many of this antioxidants would never arrive inside the cell when you take them by mouth. That will do nothing, so all these people taking antioxidants by mouth and thinking that will increase lifespan, it’s never going to happen. It has to be orchestrated. We need oxidation at certain times, we need it in certain places, and we need it to be orchestrated with certain places. Where, when and how it’s done, is what will determine whether that oxidation is good for us or bad for us and whether it will up regulate repair mechanisms. And just taking antioxidants as a blanket to reduce damage doesn’t mean it’s going to be healthy. And very often it’s not. Which is why there are quite a few studies that show that people who take certain antioxidants increase their risk of cancer. When these studies came out many years ago, they were blasted with, “It’s not true!” Well it was true. It’s just that they didn’t want to see it. That’s because of a narrow view of what health is and what life is. Life is in the orchestration.
SHELLEY – So you’re focusing on how important and fundamental it is in the body to have clear signals of repair in all our cells. Maintain those cells. Repair, repair.
RON ROSEDALE – And the most powerful of those signals that’s going to orchestrate repair are going to come from nutrient sensing pathways that are common to virtually all animal life. We know that one of the major pathways is the insulin pathway. That’s been known for 20 some years now. And more recently we know that perhaps even more importantly, the mTOR pathway feeds into the TOR pathway. And TOR uses all this information to decide whether to up regulate maintenance and repair or flip to the other side and initiate cell division. So it’s an extremely important pathway that needs to be accounted for in a person’s diet.













1 comment for “Ron Rosedale – Fiber – Need It or Not?”